A solution that contains the maximum amount of solute than can be dissolved in a given amount of solvent. 2 Show answers Another question on World Languages.

Solutions Solvent And Solute Teaching Chemistry Interactive Science Notebook Chemistry Classroom
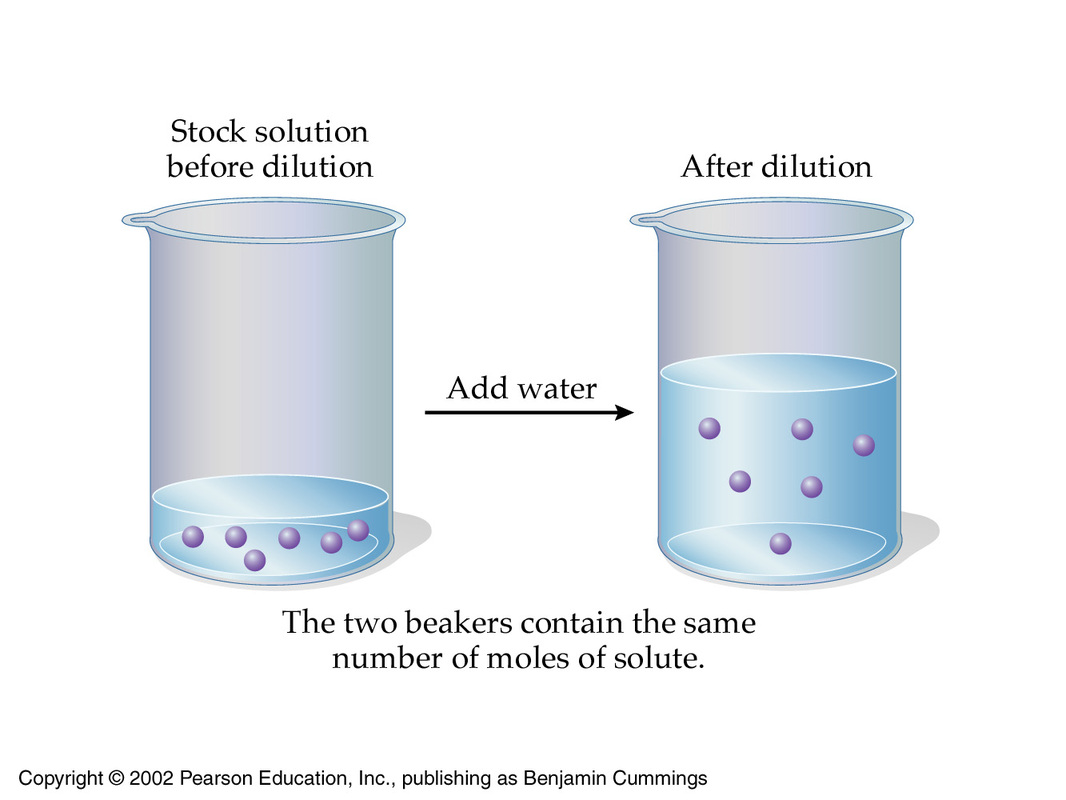
For instance if your lemonade was too tart you would add more water to decrease the concentration.

. 816 Percent by volume volume of solute volume of solution 100 817 40 mL ethanol 240 mL solution 100 818. Test a more concentrated citric acid solution. 811 Percent by mass mass of solute mass of solution 100.
The solute is the minor part of the solution. Two or more substances that are not able to mix completely. You can make a concentrated solution more dilute by adding solvent.
2 Add NaCl or other salt to shield the charges on each protein from those on. When a solution is saturated. The higher the temperature the more soluble most gases are in water.
ML of ethanol and adding enough water to make 240. Question 2multiple choice worth 5 points mc in which instance would it be most to include headings or sub headings in an essay. Increasing the solvent would decrease the concentration.
You need to add more solute to the solvent. An atom that has a positive or negative charge. Based on the kind of substance that has been dissolved the particles of a solute can be ions atoms or molecules.
How can a solution be made more concentrated. Particles from a mixture mixing together but then eventually settling down to the bottom. Salter more concentrated add more.
Molarity number of moles volume of solution L For us this would be. The definition of dissolve is. Glass is transparent to visibile light under normal conditions.
Two reasonable answers. Add another toothpick scoop of citric acid to the citric acid cup. When the solute in a solution is a solid a convenient way to express the concentration is a mass percent massmass which is the grams of solute per 100 g of solution.
Sugar and dissolve it in the water to make a concentrated solution. A solution that can hold more solute is called. Two or more substances mixing completely so that it appears as one.
A homogeneous uniform mixture of two or more substances. Add 1 drop of this more. If you have a concentrated solution can you make it more concentrated by continuing to add scoops of Kool-aid powder to the solution.
This is incorrect because the answer should have been 256 M. If you raise the temperature of a saturated solution you can usually add more solute and make the solution even more concentrated. You are given a small beaker of solution at room temp.
Molarity 004 moles 06 moles 0100 L 0150 L 256 M Adding the concentrations together and then dividing the resulting value by 2 gives 04 4 2 44 2 22 M. Gently swirl until the citric acid dissolves. 1 Show answers Another question on Physics.
1 Add HCl or N aOH to change the p H until these groups are mostly positively or nregatively charged causing them to no longer favor aggregation. When a solution cannot dissolve more solute it is called. How do you think the color will change if you add one drop of a more concentrated citric acid solution to the universal indicator in the next well.
A solute dissolves in a solvent to form a solution. If you have a solution of a chemical dissolved in water or any other solvent like ethanol for example you can make the solution more concentrated either by adding more of the chemical or by removing some of the solvent. Is this statement true or false.
If a solution is made by taking 40. However at extremely high intensities glass will absorb most of the light incident upon it. Yes the more mineral you add the more concentrated it is.
Increasing the solute would increase the concentration. World Languages 25062019 0400. A solution in which more solute can be dissolved.
Check all that apply. To make a given solution more concentrated you will have to add more solute. A solution has two parts a solvent and a solute.
Suppose that a solution was prepared by dissolving 250 g of sugar into 100 g of water. You add a bit of solute to the solution and it dissolves. As you cool a saturated solution from high temperature to low temperature solids start to crystallize out of solution.
The simplest way to change the concentration would be to change the amount of solute or solvent in the solution. You can make a concentrated solution more dilute by adding solvent. This works through a.
ML of solution the percent by volume is. The skeletal remains of diatoms aquatic organisms small creatures living in water with hard shells made of silica.
Ml Of Solution Has A Concentration Of 3 0 M How Much Water Needs To Be Added To Dilute The Solution To 1 9 M Socratic


0 Comments